You may have suffered from sleep apnea for years and not known it. When a doctor finally diagnosed you with sleep apnea, they prescribed a continuous positive airway pressure (CPAP) device. The device took some getting used to, but you were getting the best sleep in memory. Your daytime concentration issues ended. You learned to love your CPAP.
Then you heard that your brand of CPAP device was dangerous. But the maker would provide you with a new safe CPAP machine. Then you heard you would have to wait 12–18 months to get your new CPAP. You can’t wait that long, so you decide to buy a new one. Unfortunately, because of supply chain issues and the fact that every other person in your situation is thinking the same thing, none is available.
What are you going to do now? There are millions of CPAP users in the same situation you’re in. This article discusses the steps to take to make the best of this situation. It also discusses how to be compensated by a company that knowingly left you at risk from these dangerous machines. That’s right, the manufacturer knew of the risk for at least three years before they warned you.
History of CPAP Therapy
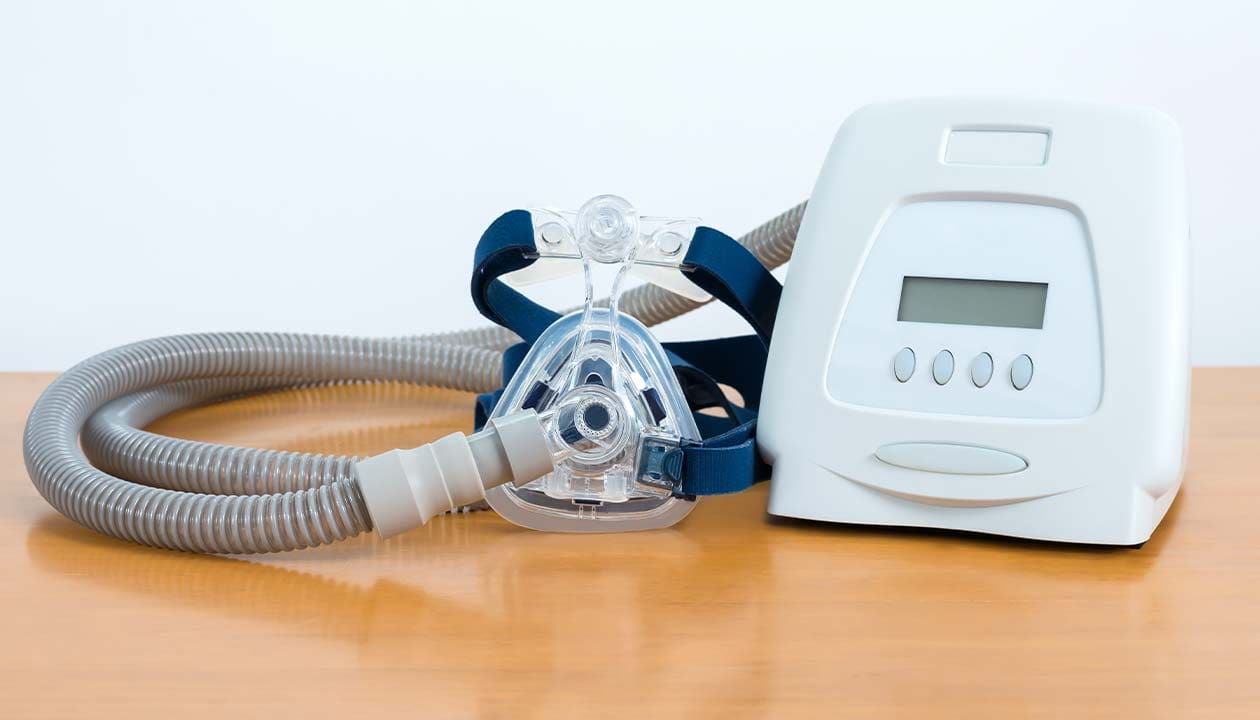
CPAP therapy had been around for decades when, in 1980, an Australian doctor tested it on a sleep apnea patient. CPAP proved to be incredibly successful in treating sleep apnea. In 1985, Respironics made CPAP machines for sleep apnea available for American patients. These first devices were extremely uncomfortable and loud. They used oxygen tanks. And there was only one mask to choose from.
Over the years, scientists made many advances with CPAP devices. A large selection of different masks became available. You can now find a mask that’s comfortable for you. While most masks cover the mouth and nose, you now have the option of a nasal only mask.
New types of CPAP came on to the market — like BiPAP. CPAP machines provide the same air pressure whether you’re breathing in or out. BiPAP devices provide higher pressure for inhales and lower pressure for exhales. Some devices automatically adjust based on your breathing patterns.
Modern CPAP devices have amazing features. Some devices have modems that provide continuous data to your doctor. Devices come with color touchscreens. There are no oxygen tanks. And the devices are much smaller than the bulky devices that first came out. The devices are about the size of a shoebox. Devices made for travel are smaller.
One complaint many CPAP users had was that daily cleanings were necessary. If you don’t keep your device clean, it can make you sick because of the accumulation of mold and bacteria. All you need to do to clean it is disassemble it and clean each part. People are too busy in the 21st century to hand wash a machine once per day. Some smart business people developed solutions to the hassle of daily cleanings.
Ozone-based and UV-light cleaning devices came on the market. These solved the cleaning hassle issue.
Another issue that was solved by technical advances was the noise. Patients use these devices while sleeping. Loud noises make it difficult to sleep. Modern CPAP machines are much quieter than the early CPAP devices. Different device-makers use different technologies for noise reduction.
Ironically, the sound abatement technology is at the center of the current problem identified in certain devices made by Philips Electronics. In 2008, Philips, a global electronic giant, bought Respironics, the makers of the first CPAP devices sold in the US.
For at least the past decade, users have been complaining about unexpected side effects from some of the Philips CPAP machines. These patients have also noticed tiny black particles in the machine’s air pathways.
Only recently did we learn the source of the black particles is the polyester-based polyurethane sound abatement foam. This foam degrades to produce the black particles. The foam contains some incredibly dangerous chemicals. It’s believed these chemicals are what’s causing the many severe symptoms patients have complained of. Many of these patients have brought lawsuits.
Surprisingly, Philips claims they haven’t authorized ozone-based and UV-light cleaning devices. Philips says the only approved cleaning method is to disassemble and hand wash the parts. Philips asserts that ozone-based cleaning speeds up the degradation of the noise-reducing foam.
So, clean your device to keep from getting sick. But now Philips says, if you clean the device in the wrong way, it will also make you sick. Obviously, plaintiffs’ attorneys aren’t buying Philips’ claim.
Brand Names of CPAP Machines
Unlike drugs, device brands don’t have underlying chemical names. For example, a once popular gastrointestinal reflux medication, Ranitidine, the public knew the drug by its brand name; Zantac. With CPAP machines, you have devices made by many manufacturers that have different model names. Three of the largest manufacturers are Philips Respironics, ResMed, and Fisher & Paykel.
Instead of listing the many model names of each CPAP manufacturer, we’ll focus on the dangerous machines in the recall section below.
Overview of Research Findings

Research shows the treatment effectiveness and cost effectiveness of CPAP therapy. Experts consider CPAP therapy as the gold standard for treating sleep apnea. CPAP therapy works when used as prescribed and when the device is safe.
Unsafe devices, like some devices made by Philips, present a difficult issue. A device that should provide better health provides worse health. How bad is it? In short, laypeople’s terms, it is terrible.
The dangerous chemicals in the sound abatement foam in these Philips devices include:
- Diethylene glycol
- Dimethyl diazene (Azoxymethane)
- Phenol, 2,6-bis (1,1-dimethylethyl)-4- (1-methylpropyl)
- Toluene diamine
- Toluene diisocyanate
These chemicals cause everything from eye irritation to cancer to death by poisoning. But there are only trace amounts of these chemicals in those tiny black particles you see. Can it really be that harmful? Yes. Courts are now evaluating cases involving the following health issues:
- Severe Ear Inflammation
- Severe Nose Inflammation
- Severe Throat Inflammation
- Poisoning
- Acute Respiratory Distress System (ARDS)
- Pleural Effusion
- Reactive Airway Disease (RAD)
- Respiratory Failure
- Lung Damage
- Lung Disease
- Heart Attack
- Heart Failure
- Liver Damage
- Liver Disease
- Kidney/Renal Damage
- Kidney/Renal Disease
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Hematopoietic Cancer
- Kidney Cancer
- Leukemia
- Liver Cancer
- Lung Cancer
- Lymphatic Cancer
- Multiple Myeloma
- Nasal Cancer
- Non-Hodgkin Lymphoma
- Papillary Carcinoma
- Prostate Cancer
- Rectal Cancer
- Stomach Cancer
- Testicular Cancer
- Thyroid Cancer
You can think of these black particles like cigarette smoke. Smoking a single cigarette won’t harm you. But continuously smoking over time allows dangerous chemicals to build up in your system. In the same manner, continuing to inhale the dangerous particles in these CPAP machines causes dangerous chemicals to build up in your system. And, these chemicals are probably more dangerous than cigarette smoke.
Over the past year, the FDA has received 21,000 complaints about these devices. The FDA has also recorded 124 deaths from the use of these devices.
Recalls

On April 26, 2021, Philips issued a voluntary recall for many of its CPAP devices, BiPAP devices, and ventilators. They updated the list to include the following devices:
- A-Series BiPAP A30
- A-Series BiPAP A40 (ventilator)
- A-Series BiPAP Hybrid A30
- A-Series BiPAP V30 Auto (ventilator)
- C-Series ASV (ventilator)
- C-Series S/T and AVAPS
- DreamStation
- DreamStation ASV
- DreamStation Go
- DreamStation ST, AVAPS
- Dorma 400
- Dorma 500
- E30
- Garbin Plus, Aeris, LifeVent (ventilator)
- OmniLab Advanced+
- REMstar SE Auto
- SystemOne ASV4
- SystemOne (Q-Series)
- Trilogy 100 (ventilator)
- Trilogy 200 (ventilator)
The Food and Drug Administration (FDA) assigned this recall its highest risk level: a Class I recall. That means the FDA views the recalled devices as a “situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
Unfortunately, there have been some issues with the recall. At first, Philips was going to replace the defective CPAP machines with silicone-based sound abatement. But, the FDA determined this solution is also unsafe.
Philips had been manufacturing 55,000 replacement CPAP machines per week. Even at that rate, it would take almost 18 months to replace all the defective CPAP machines. Global supply chain issues have also hampered production. Many patients don’t have 18 months to wait for relief from sleep apnea.
Patients have been trying to get CPAP devices from other manufacturers. But ResMed, Philips’ largest competitor, reports it can’t keep up with demand coming from those trying to replace their Philips devices.
The most important thing you, a CPAP user, can do is to register your device with Philips. You can’t receive a replacement unless it’s registered. If you haven’t already registered, Philips has a special web page for you to get on the recall list.
Now that you’re on the recall list, your next step needs to be scheduling an appointment with your doctor. You need to decide, with your doctor, whether you should continue using the device while waiting for a new one. If not, you’ll want to discuss alternative treatments. Understand your doctor is probably as frustrated by the issue with Philips as you are.
Physician Sentiment
Doctors are in a tough situation. Dr. Andrey Zinchuck of the Advanced Apnea Management program at Yale University, says they don’t know the actual risk since there’s some evidence that the harmful particle emissions taper off after the initial use. After the initial use? Philips blames the problem on accelerated degradation caused by ozone cleaners.
Degradation suggests the breakdown occurs over time. So, the initial use wouldn’t be the time for concern. Dr. Zinchuck also says they just don’t know how dangerous these devices may be. There was insufficient evidence to make a determination at the time of the interview in April 2022.
In August, 2021, Dr. Timothy I. Morgenthaler, a sleep medicine specialist at the Mayo Clinic in Rochester, Minnesota, told the New York Times doctors need a timeline for when they can expect replacements. He also said doctors need a way to quantify the risk.
With some of America’s leading doctors frustrated by the situation, ordinary healthcare providers don’t know what to do. The American Academy of Sleep Medicine (AASM) has advised doctors to discuss guidance with their risk management team or attorneys. These doctors want to help, but don’t want to risk getting into trouble themselves.
The AASM also notes that Philips says the foam related complaint rate in 2020 was only 0.03%. That’s interesting because the FDA shows 21,000 complaints and 124 deaths in 2021.
Some doctors are considering alternative treatments while patients wait for their new and improved CPAP machines. Dr. Henry Yaggi, a pulmonologist and director of the Yale Center for Sleep Medicine, suggests the following alternative treatments:
- Oral appliances dentists have been using to treat sleep apnea
- Lifestyle changes such as quitting smoking and losing weight
- Upper airway exercises
- Not sleeping on your back
- The Inspire implanted device that manipulates nerve impulses to control sleep apnea
These are options you can try, but you really want your new CPAP machine. You need to decide after consulting with your doctor if you should continue to use a recalled CPAP machine while waiting for a new one.
After you talk to your doctor, you’ll want to contact an attorney to discuss your situation with Philips. One of the primary questions the attorney will have for you is about the results of any tests the doctor performed on you. Once you read about the lawsuit below, you’ll probably be ready to bring your own claim against Philips.
History of Lawsuit

Patients have filed hundreds of individual lawsuits and class actions against Philips for these defective machines. Courts have consolidated these cases in the federal court for the Western District of Pennsylvania in Pittsburg. Attorneys call this consolidation procedure multi-district litigation (MDL).
The short explanation of MDL is that courts transfer all their Philips defective CPAP lawsuits to one court for rulings on pretrial motions, discovery, and alternative dispute resolution. The judge appointed a mediator in July. If there’s no settlement, selected cases will go to trial.
These bellwether cases will give the parties not yet going to trial an idea of what they can expect should their cases go to trial. Courts hope these results will help bring a settlement among the many parties. If there’s no settlement, a case goes back to the original district where the plaintiffs filed their case. Then, the case gets its own trial.
One of the most interesting developments to come from the discovery process so far is that attorneys found emails showing that Philips knew their CPAP machines were dangerous as early as 2018. Yet, they didn’t let you, the patient, in on this secret until April 2021 — three years later! It’s time for you to join the many, and receive compensation for what Philips knowingly did to you.

