Surgical staplers and staples have come a long way since their invention in the early 19th century. Today, these tools are essential for many types of surgery and are generally considered safe and reliable. Surgical staplers and staples are commonly used in a variety of medical procedures. They are typically made of stainless steel or titanium and are designed to close up wounds quickly and effectively.
The bulk of the surgical stapler market in the United States (US) is controlled by Medtronic, which bought Covidien in 2014, while Johnson & Johnson affiliate, Ethicon, controls the surgical stapler market. In fact, Medtronic and Ethicon produce 4 out of 5 surgical staplers used in American hospitals.
As with any medical device, there is always some risk of complication. These devices have been the subject of a number of complaints and lawsuits in recent years. As a result, many doctors are now taking a closer look at these devices and their potential risks.
While most surgeons still believe that surgical staplers and staples are safe and effective, there is some concern among physicians about the potential complications. More victims are coming forward with injuries related to these devices. If you’re among those harmed, Join the Many can help you better understand how to exercise your legal rights and get the justice you deserve.
History of Surgical Staplers and Staples
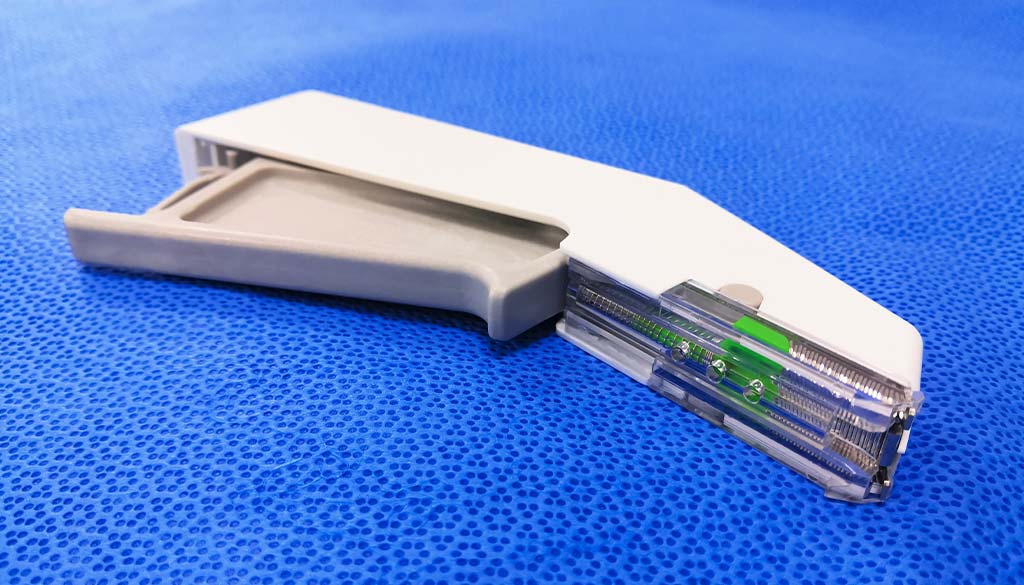
Surgical staplers and staples are used instead of sutures to close surgical incisions and wounds. Staplers come in different sizes and shapes, but all work by passing metal staples through the layers of skin and tissue below the surface. The ends of the staples are then bent over to hold the tissues together while they heal. Staples can also be used for other purposes, such as closing off an Ileostomy or Colostomy bag.
Unfortunately, surgical staplers are prone to malfunction resulting in serious patient injuries. Surgical stapler injuries include:
- Internal organs and tissue that have been torn or damaged
- A possibly fatal reaction to an infection called sepsis
- Increased risk of cancer relapse
- Fistulas forming between organs or other body tissues
- Internal bleeding
- Death
Overview of Research Findings
Every year, medical errors lead to the death of hundreds of thousands of people around the world. In fact, one study estimates that medical errors are the third leading cause of death in the US. And given the high volume of stapler use, many surgeons are likely to encounter surgical stapler malfunction in their careers.
Research by Benham et al. compared surgical device malfunction complaints in the Manufacturer and User Facility Device Experience (MAUDE) database to those in the nonpublic alternative summary report and found that:
“There were 283,308 (72%) general surgical device malfunctions in MAUDE and 109,954 (28%) in ASR. Reports increased annually in ASR versus MAUDE, particularly for surgical staplers and clip devices (p < 0.05). ASR contained approximately 80% of these reports; MAUDE 20%. In MAUDE, 42.9% of surgical device malfunctions and 20.2% of stapler/clip malfunctions resulted in patient injury or death. ASR listed no injury or death information. ASR contained a significant portion of surgical device malfunctions hidden from public scrutiny.”
Recalls and Updates
For clarity on the frequency of surgical stapler malfunctions, you can simply look at the volume of adverse events reported to the FDA through its MAUDE database. During the first ten years, the FDA received 22,204 adverse event reports that included 2,180 injuries and 112 deaths.
More recently, research on MAUDE shows that for the period January 2018 to December 2020:
- 883 adverse events were reported for Medtronic
- 353 were reported for Ethicon
- Thirty-five events were reported for Intuitive.
- The misfire rate for Medtronic was 0.04%, and for Ethicon was 0.02%
- The most commonly reported event for Medtronic was a failure to fire, followed by a misfire.
- For Ethicon, the most common event was a failure to fire, followed by mechanical problems. The most common event with the Intuitive stapler was a leak and bleeding from the staple line.
Before the May 2018 recall, Medtronic reported no less than 5 injuries. And corporations frequently discover manufacturing flaws that can potentially cause injuries. In a 2019 recall, Medtronic also reported more than 3 million staplers reloads that missed components. These missing components could cause staples to fail, resulting in “bleeding, anastomotic leak, peritonitis, or pneumothorax, which can result in the possibility for infection and/or sepsis.” Other flaws resulting in recalls can potentially induce bleeding or spilling of abdominal contents into nearby body cavities.
FDA Investigation and Guidance
According to a report from the U.S. Food and Drug Administration (FDA), there have been more than 41,000 reports of a surgical stapler and staples injuries, malfunctions, and deaths since 2010. This includes more than 9,000 reports of serious injuries and almost 1,400 reports of death.
In July 2019, the FDA started investigating reports of serious injuries and deaths associated with certain surgical staplers and staples for use in internal organs and other tissue.
In October 2021, the FDA released guidance for surgical stapler safety. The guidance provides recommendations for manufacturers of surgical staplers and staples and healthcare facilities that use these devices. The FDA aims to reduce the risk of serious complications associated with surgical staplers and staples.
The guidance is intended to help ensure that the labeling for these devices contains consistent and accurate information on the indications, contraindications, warnings, and precautions associated with their use. This guidance finalizes the labeling recommendations contained in the July 2017 draft guidance of the same title.
General Physician Sentiment
For years, the risks of surgical staplers have been underreported. In fact, it wasn’t until recently that the FDA released a statement admitting that they had been receiving hundreds of reports of complications associated with these devices. Between 2011 and 2018, the public database was updated with 41,000 additional malfunctions, while 56,000 more were concealed in the hidden database.
In 2016, Kaiser Health News reported that the FDA added less than 100 injuries relating to surgical staplers to MAUDE. But there were almost 10 000 reports in the hidden database as an alternative summary report. After this report, the FDA made a concerted effort to release the hidden report and tighten surgical stapler regulations.
In response, the FDA, through their chief medical officer, Dr. William Maisel, stated that:
“The increasing reliance on surgical staplers by surgeons to perform more procedures that are minimally invasive, together with the agency’s analysis of adverse events associated with surgical staplers and implantable staples, prompted the FDA to increase regulatory oversight of these devices while continuing to educate healthcare providers and patients about their benefits and risks.”
But, experts highlight that despite recalls, millions of surgeries are done with staplers, and they are improving all the time. Dr David Urbach, Medical Director at the Women’s College Hospital, cautions:
“It’s really important to know that surgical staplers are used routinely every day throughout the country, throughout the world. There are tens of thousands of people having surgery using surgical staplers for internal use. And by and large, they’re very, very reliable, and they’re very useful for surgical procedures.”
Brief Lawsuit History
The most well-known lawsuit concerning surgical staplers and staples is arguably that of Kuhlmann v Ethicon.
A jury awarded almost $80 million in damages to Florence Kuhlmann, a woman who claimed she suffered serious injuries after hemorrhoid surgery. The jury found that the Ethicon surgical staplers and staples used in the procedure were defective and that the manufacturer failed to warn patients about the risks associated with the devices.
This case highlights the importance of choosing a qualified surgeon who is experienced in performing this type of surgery. It also underscores the need for patients to be fully informed of the risks and benefits of any procedure before undergoing treatment. The amount was reduced to $19.6 million on appeal.
The lawsuit is one of many surgical stapler cases that Covidien and other manufacturers are facing. Similar claims are made in each of these cases: that the devices malfunctions, fail to fire, or issue misshapen staples. This can result in life-threatening internal injuries and organ tears—were caused by design flaws. We briefly highlight the latest ones below. We briefly highlight the latest ones below.
Kimberley Bell v. Covidien LP and 3 Others
Recently, a North Carolina woman, Kimberly Bell, filed a product liability lawsuit against Medtronic and its Covidien subsidiary. Bell underwent surgery that was intended to be relatively straightforward. But something went wrong. The Endo GIA stapler malfunctioned during the surgery.
This caused a tear in the surgical area requiring extensive medical care and prolonged hospitalization and extensive medical care. Bell is now suing the stapler manufacturer, Covidien LP, for damages. In her lawsuit, she claims that the company failed to properly design and test the stapler, leading to her injuries.
William Bruce Jones & Kathleen Gail Jones v Medtronic, Inc. and 5 Others
In March 2020, William Bruce Jones and his wife, Kathleen, filed a product liability lawsuit against Medtronic in the U.S. District Court of Minnesota. The lawsuit claims that a Covidien surgical stapler broke during surgery and caused an abdominal leak. This led to an infection and the need for a colostomy. According to the complaint, the stapler was defective and did not function as intended.
The Joneses allege that they have incurred significant medical expenses as a result of the incident and are seeking compensation for, amongst others, medical and incidental expenses, past and future loss of earnings, pain and suffering, disfigurement, and/or disability. Medtronic has not yet filed a response to the complaint.
Derick Paul Smith v. Covidien LP and Medtronic, Inc.
Louisiana man Derick Paul Smith also filed a product liability lawsuit, having suffered severe internal injuries after a surgeon used a Covidien Tri-Staple Endo GIA Surgical Stapling Device during laparoscopic surgery. The lawsuit, filed in the U.S. District Court for the Eastern District of Louisiana, claims that the device was defective and that Covidien failed to warn of the risks associated with its use.
As a result of the defects, Smith had to undergo multiple surgical procedures and incurred significant medical expenses. The lawsuit is seeking damages for Smith’s injuries and punitive damages.
Surgical Staples Class Action Lawsuit in Canada
Also concerning surgical staplers, a class action lawsuit has been filed in Canada against Medtronic and its Covidien subsidiary over allegedly defective surgical staplers. The plaintiffs allege that the staplers have caused serious injuries, including internal bleeding, organ damage, and even death.
Medtronic is one of the world’s largest manufacturers of surgical staplers, and Covidien is one of its subsidiaries. The lawsuit alleges that the companies knew or should have known about the defects in the staplers, but failed to warn consumers or take appropriate action.
What are the Damages in Surgical Staplers and Staples Lawsuits?
Did you experience a medical issue with surgical staples following a procedure in 2011? Financial compensation may be available through a medical malpractice lawsuit or product liability claim due to the following:
- staple defects
- staplers misfiring or failing to fire at all
- internal injuries, bleeding, and organ perforation
- wrongful death
Patients can bring a surgical stapler product liability lawsuit against the device’s manufacturer if they’ve been injured by a malfunctioning surgical stapler and get financial compensation for their injuries. Compensation can be claimed for the following
- medical expenditures
- funeral expenses for deaths as a result of stapler injuries
- physical and mental pain and suffering
- lost wages
- loss of earning capacity
- loss of enjoyment of life

